FOR PHYSICIANS
SEARCH
Limitations of the Harmony® Test
NIPT (non-invasive prenatal test) methods such as the Harmony® Test are used to detect chromosomal disorders (trisomies 21, 18, 13, and X/Y). What makes this method special is that it is non-invasive: only the mother’s blood is tested.
A NIPT method, however, cannot replace an ultrasound scan in the first trimester. First trimester screening and/or ultrasound scan towards the end of the first trimester can detect possible disorders in the fetal organs and is still strongly recommended.
cffDNA-based NIPT methods are not yet able to detect chromosomal mosaicism, partial trisomies or translocations.
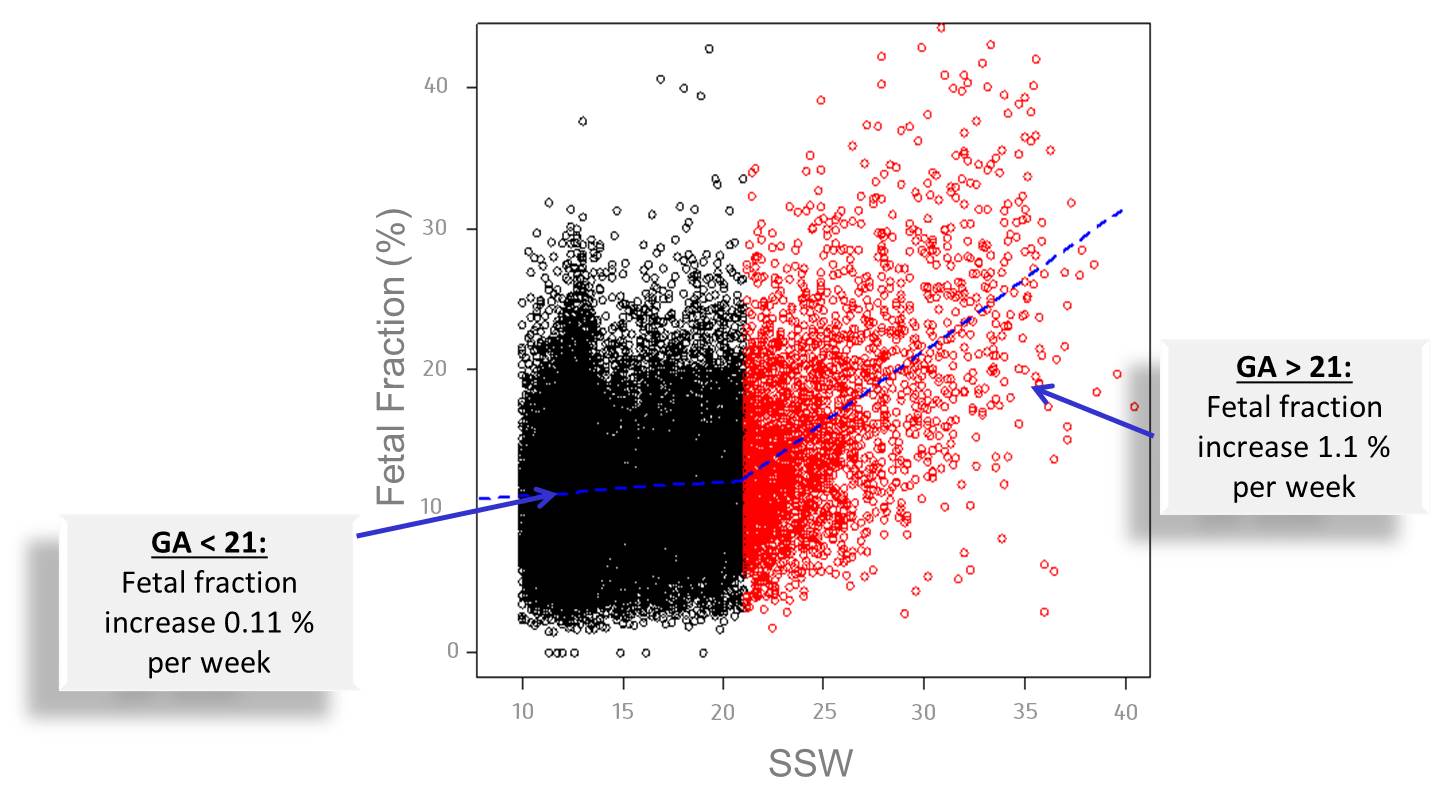
In order to ensure the detection of a chromosomal disorder in the unborn child, the proportion of cell-free fetal DNA (cffDNA) (“Fetal Fraction”) in the mother’s blood must be at least 4% 1. The Harmony® Test can be performed after pregnancy week 10+0 because the proportion of fetal DNA in the mother’s blood rises with the length of the pregnancy (see figure below).
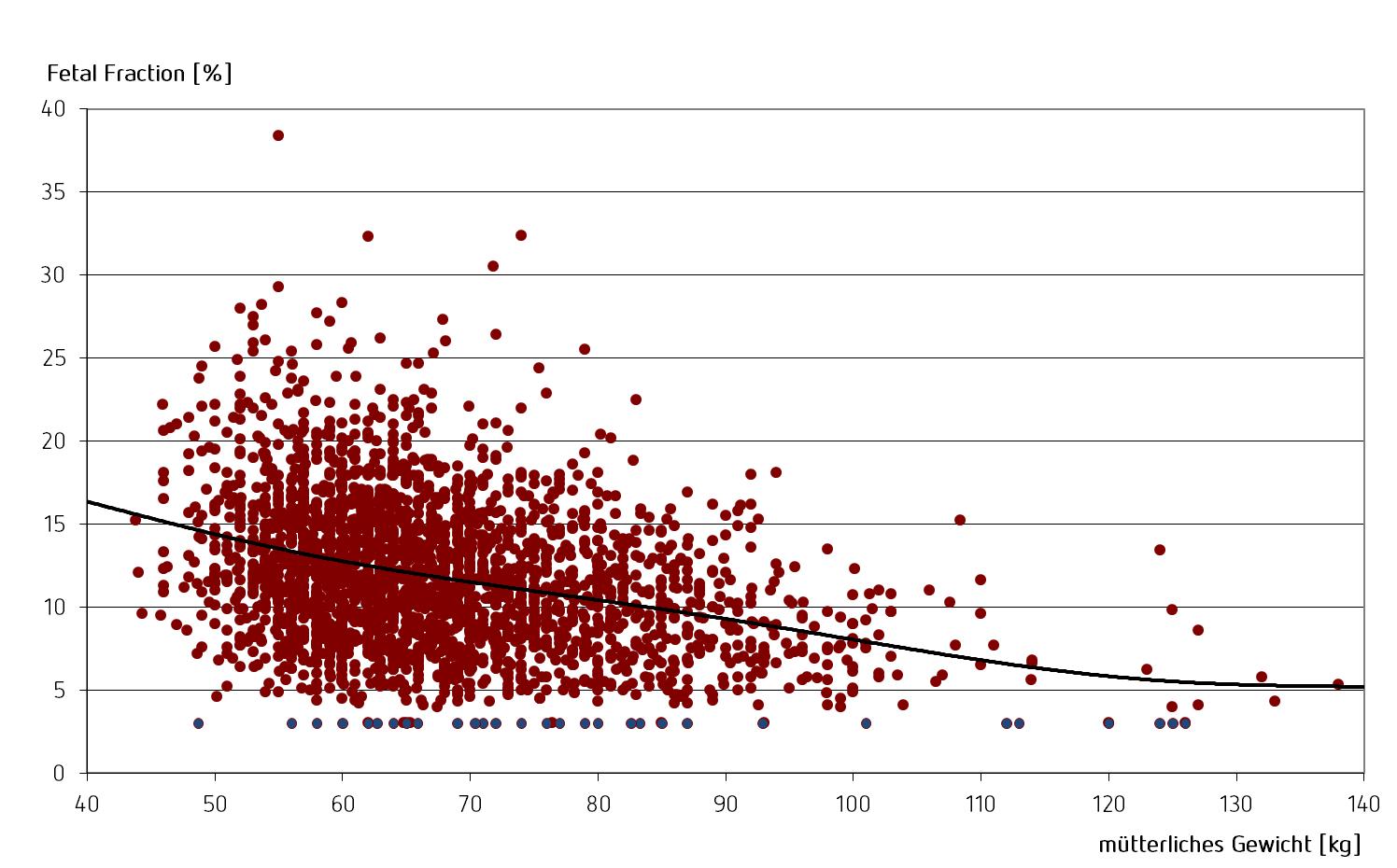
There is also a correlation between the mother’s weight and the “Fetal Fraction”: the higher the mother’s weight, the lower the proportion of fetal DNA in the mother’s blood.
The Harmony® Test can be performed irrespective of the manner of conception or origin of the ovum. Due to specialized bioinformatics analyses, however, by filling out the test requisition form it must be informed about an egg donation when the test is requested. If an egg donation is kept secret, the Harmony® Test is not able to deliver a result.
In the case of a vanishing twin, we recommend performing the Harmony® test no earlier than the 15th week of pregnancy, or at least eight weeks after the death of the second fetus10. This is because the placenta of the deceased twin often persists for several weeks, continuing to release cell-free DNA.
The Harmony® Test is not validated for use in pregnancies with maternal aneuploidy, transplant or malignancy.
| Trisomy | Detection rate | False positive rate | Number of trisomy cases | Number of euploid gemini pregnancies |
| 21 | ||||
| 18 | ||||
| 13 |
| Singleton pregnancies | Twin pregnancies | More than two fetuses | |
| Harmony® T21/T18/T13 | |||
| Harmony® T21/18/13 + X/Y-Analysis | |||
| Sex determination |
In a very small number of cases, the Harmony® Test cannot be performed successfully5 6. This is usually because the proportion of cell-free fetal DNA (cffDNA) in the blood is too low for a result to be obtained. In such a case we recommend blood be taken again at a later point (after 2 weeks) and the Harmony® Test be repeated. In our experience, 50% of the second submissions can then be succesfully evaluated. In case a test result cannot be obtained, a decision has to be made with the patient as to whether further first trimester screening or possibly an invasive procedure should be performed.
Other reasons for test failure include the existence of an egg donation about which was not informed. With the subsequent information of an egg donation, the Harmony® Test can usually obtain a result.
Transplantations or previously unknown (and in general benign) maternal chromosomal disorders can also lead to a failure of the test. For these cases the Harmony® Test is not validated and should not be performed or repeated.
Despite significant improvements over former non-invasive tests, the detection rate for the Harmony® Prenatal Test is not 100%. False-positive results may occure. Therefore the Harmony® Prenatal Test should be regarded as a screening test and not a diagnostic test. A positive (abnormal) result should therefore always be confirmed by a second, diagnostic method (chorionic villus sampling or amniotic puncture) with a subsequent chromosome analysis.
False-negative results are also possible. This is more common for trisomy 13.
The Harmony® Test detects around 30 out of 32 trisomy 13 cases in its current version. A summary of all previous studies on trisomy 13 revealed an average detection rate of 93,8% 7. Trisomy 13 is, however, relatively easy to detect in an ultrasound scan during first trimester screening.
- Ashoor G, Poon L, Syngelaki A, Mosimann B, Nicolaides KH. Fetal Fraction in Maternal Plasma Cell-Free DNA at 11–13 Weeks’ Gestation: Effect of Maternal and Fetal Factors. Fetal Diagn Ther 2012;31:237-243. ↩
- Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013 Jul;33(7):662-6. doi: 10.1002/pd.4119. Epub 2013 May 9. ↩
- Bevilacqua E, Gil MM, Nicolaides KH, Ordoñez E, Cirigliano V, Dierickx H, Willems PJ, Jani JC. Performance of screening for aneuploidies by cell-free DNA analysis of maternal blood in twin pregnancies. Ultrasound Obstet Gynecol. 2015 Jan;45(1):61-6. doi: 10.1002/uog.14690. Epub 2014 Dec 4. ↩
- Gil MM, Quezada MS, Bregant B, Syngelaki A, Nicolaides KH. Cell-free DNA analysis for trisomy risk assessment in first-trimester twin pregnancies. Fetal Diagn Ther. 2014;35(3):204-11. doi: 10.1159/000356495. Epub 2013 Nov 15. ↩
- Lüthgens K, Grati FR, Sinzel M, Häbig K, Kagan KO. Confirmation rate of cell free DNA screening for sex chromosomal abnormalities according to the method of confirmatory testing. Prenat Diagn. 2020 Aug 17. doi: 10.1002/pd.5814. Epub ahead of print. PMID: 32804406. ↩
- Kagan, K. O., Sroka, F., Sonek, J., Abele, H., Lüthgens, K., Schmid, M., Wagner, P., Brucker, S., Wallwiener, D. and Hoopmann, M. (), First trimester screening based on ultrasound and cfDNA vs. first-trimester combined screening – a randomized controlled study. Ultrasound Obstet Gynecol. Accepted Author Manuscript. doi:10.1002/uog.18905 ↩
- Stokowski R, Wang E, White K, Batey A, Jacobsson B, Brar H, Balanarasimha M, Hollemon D, Sparks A, Nicolaides K, Musci TJ.: Clinical performance of non-invasive prenatal testing (NIPT) using targeted cell-free DNA analysis in maternal plasma with microarrays or next generation sequencing (NGS) is consistent across multiple controlled clinical studies. Prenat Diagn. 2015 Sep 1 ↩
- Schmid M et al. Prenatal Screening for 22q11.2 Deletion Using a Targeted Microarray-Based Cell-Free DNA Test.Fetal Diagn Ther. 2017 Nov 8, E-pub ahead of print ↩
- Judah H. et al.: Cell-free DNA testing of maternal blood in screening for trisomies in twin pregnancy: updated cohort study at 10–14 weeks and metaanalysis. Ultrasound Obstet Gynecol 2021; 58: 178–189 ↩
- van Eekhout JCA, Bax CJ, Schuurman LVP, Becking EC, van der Ven AJEM, Van Opstal D, Boon EMJ, Macville MVE, Bekker MN, Galjaard RJH; Dutch NIPT Consortium. Performance of non-invasive prenatal testing in vanishing-twin and multiple pregnancies: results of TRIDENT-2 study. Ultrasound Obstet Gynecol. 2025 Sep 6. doi: 10.1002/uog.70015. Epub ahead of print. PMID: 40913805.
FOR PHYSICIANS
SEARCH
Limitations of the Harmony® Test
NIPT (non-invasive prenatal test) methods such as the Harmony® Test are used to detect chromosomal disorders (trisomies 21, 18, 13, and X/Y). What makes this method special is that it is non-invasive: only the mother’s blood is tested.
A NIPT method, however, cannot replace an ultrasound scan in the first trimester. First trimester screening and/or ultrasound scan towards the end of the first trimester can detect possible disorders in the fetal organs and is still strongly recommended.
cffDNA-based NIPT methods are not yet able to detect chromosomal mosaicism, partial trisomies or translocations.
In order to ensure the detection of a chromosomal disorder in the unborn child, the proportion of cell-free fetal DNA (cffDNA) (“Fetal Fraction”) in the mother’s blood must be at least 4% 1. The Harmony® Test can be performed after pregnancy week 10+0 because the proportion of fetal DNA in the mother’s blood rises with the length of the pregnancy (see figure below).
There is also a correlation between the mother’s weight and the “Fetal Fraction”: the higher the mother’s weight, the lower the proportion of fetal DNA in the mother’s blood.
The Harmony® Test can be performed irrespective of the manner of conception or origin of the ovum. Due to specialized bioinformatics analyses, however, by filling out the test requisition form it must be informed about an egg donation when the test is requested. If an egg donation is kept secret, the Harmony® Test is not able to deliver a result.
In the case of a vanishing twin, we recommend performing the Harmony® test no earlier than the 15th week of pregnancy, or at least eight weeks after the death of the second fetus9. This is because the placenta of the deceased twin often persists for several weeks, continuing to release cell-free DNA.
The Harmony® Test is not validated for use in pregnancies with maternal aneuploidy, transplant or malignancy.
The Harmony® test can be used for twin pregnancies.
A recent study by Judah et al. (2021) on twin analyses found that the detection and false-positive rates for trisomy 21 are comparable to those of singleton pregnancies9. The publication shows that cfDNA-based screening for the three relevant trisomies in Gemini pregnancies consistently outperforms first trimester screening.
Judah et al.’s meta-analysis was based on the Nicolaides group’s studies with the Harmony® test and publications with MPSS* methods. The detection and false-positive rates for noninvasive prenatal testing (NIPT) in twin pregnancies are9:
| Trisomy | Detection rate | False positive rate | Number of trisomy cases | Number of euploid gemini pregnancies |
| 21 | ||||
| 18 | ||||
| 13 |
Currently, it is not possible to make a statement about sex chromosomal disorders (X/Y analysis), such as Turner or Klinefelter syndrome, for twins.
The table below shows which Harmony® test variant is possible for which pregnancy.
| Singleton pregnancies | Twin pregnancies | More than two fetuses | |
| Harmony® T21/T18/T13 | |||
| Harmony® T21/18/13 + X/Y-Analysis | |||
| sex determination |
Sex determination is possible for both singleton and twin pregnancies. In accordance with the Genetic Diagnostics Act, the fetal sex is communicated from week 14+0 (p.m.) onwards.
In a very small number of cases, the Harmony® Test cannot be performed successfully5 6. This is usually because the proportion of cell-free fetal DNA (cffDNA) in the blood is too low for a result to be obtained. In such a case we recommend blood be taken again at a later point (after 2 weeks) and the Harmony® Test be repeated. In our experience, 50% of the second submissions can then be succesfully evaluated. In case a test result cannot be obtained, a decision has to be made with the patient as to whether further first trimester screening or possibly an invasive procedure should be performed.
Other reasons for test failure include the existence of an egg donation about which was not informed. With the subsequent information of an egg donation, the Harmony® Test can usually obtain a result.
Transplantations or previously unknown (and in general benign) maternal chromosomal disorders can also lead to a failure of the test. For these cases the Harmony® Test is not validated and should not be performed or repeated.
Despite significant improvements over former non-invasive tests, the detection rate for the Harmony® Prenatal Test is not 100%. False-positive results may occure. Therefore the Harmony® Prenatal Test should be regarded as a screening test and not a diagnostic test. A positive (abnormal) result should therefore always be confirmed by a second, diagnostic method (chorionic villus sampling or amniotic puncture) with a subsequent chromosome analysis.
False-negative results are also possible. This is more common for trisomy 13.
The Harmony® Test detects around 30 out of 32 trisomy 13 cases in its current version. A summary of all previous studies on trisomy 13 revealed an average detection rate of 93,8% 7. Trisomy 13 is, however, relatively easy to detect in an ultrasound scan during first trimester screening.
- Ashoor G, Poon L, Syngelaki A, Mosimann B, Nicolaides KH. Fetal Fraction in Maternal Plasma Cell-Free DNA at 11–13 Weeks’ Gestation: Effect of Maternal and Fetal Factors. Fetal Diagn Ther 2012;31:237-243. ↩
- Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013 Jul;33(7):662-6. doi: 10.1002/pd.4119. Epub 2013 May 9. ↩
- Bevilacqua E, Gil MM, Nicolaides KH, Ordoñez E, Cirigliano V, Dierickx H, Willems PJ, Jani JC. Performance of screening for aneuploidies by cell-free DNA analysis of maternal blood in twin pregnancies. Ultrasound Obstet Gynecol. 2015 Jan;45(1):61-6. doi: 10.1002/uog.14690. Epub 2014 Dec 4. ↩
- Gil MM, Quezada MS, Bregant B, Syngelaki A, Nicolaides KH. Cell-free DNA analysis for trisomy risk assessment in first-trimester twin pregnancies. Fetal Diagn Ther. 2014;35(3):204-11. doi: 10.1159/000356495. Epub 2013 Nov 15. ↩
- Lüthgens K, Grati FR, Sinzel M, Häbig K, Kagan KO. Confirmation rate of cell free DNA screening for sex chromosomal abnormalities according to the method of confirmatory testing. Prenat Diagn. 2020 Aug 17. doi: 10.1002/pd.5814. Epub ahead of print. PMID: 32804406. ↩
- Kagan, K. O., Sroka, F., Sonek, J., Abele, H., Lüthgens, K., Schmid, M., Wagner, P., Brucker, S., Wallwiener, D. and Hoopmann, M. (), First trimester screening based on ultrasound and cfDNA vs. first-trimester combined screening – a randomized controlled study. Ultrasound Obstet Gynecol. Accepted Author Manuscript. doi:10.1002/uog.18905 ↩
- Stokowski R, Wang E, White K, Batey A, Jacobsson B, Brar H, Balanarasimha M, Hollemon D, Sparks A, Nicolaides K, Musci TJ.: Clinical performance of non-invasive prenatal testing (NIPT) using targeted cell-free DNA analysis in maternal plasma with microarrays or next generation sequencing (NGS) is consistent across multiple controlled clinical studies. Prenat Diagn. 2015 Sep 1 ↩
- Schmid M et al. Prenatal Screening for 22q11.2 Deletion Using a Targeted Microarray-Based Cell-Free DNA Test.Fetal Diagn Ther. 2017 Nov 8, E-pub ahead of print ↩
- van Eekhout JCA, Bax CJ, Schuurman LVP, Becking EC, van der Ven AJEM, Van Opstal D, Boon EMJ, Macville MVE, Bekker MN, Galjaard RJH; Dutch NIPT Consortium. Performance of non-invasive prenatal testing in vanishing-twin and multiple pregnancies: results of TRIDENT-2 study. Ultrasound Obstet Gynecol. 2025 Sep 6. doi: 10.1002/uog.70015. Epub ahead of print. PMID: 40913805.
Limitations of the Harmony® Test
NIPT (non-invasive prenatal test) methods such as the Harmony® Test are used to detect chromosomal disorders (trisomies 21, 18, 13, and X/Y). What makes this method special is that it is non-invasive: only the mother’s blood is tested.
A NIPT method, however, cannot replace an ultrasound scan in the first trimester. First trimester screening and/or ultrasound scan towards the end of the first trimester can detect possible disorders in the fetal organs and is still strongly recommended.
cffDNA-based NIPT methods are not yet able to detect chromosomal mosaicism, partial trisomies or translocations.
In order to ensure the detection of a chromosomal disorder in the unborn child, the proportion of cell-free fetal DNA (cffDNA) (“Fetal Fraction”) in the mother’s blood must be at least 4% 1. The Harmony® Test can be performed after pregnancy week 10+0 because the proportion of fetal DNA in the mother’s blood rises with the length of the pregnancy (see figure below).
There is also a correlation between the mother’s weight and the “Fetal Fraction”: the higher the mother’s weight, the lower the proportion of fetal DNA in the mother’s blood.
The Harmony® Test can be performed irrespective of the manner of conception or origin of the ovum. Due to specialized bioinformatics analyses, however, by filling out the test requisition form it must be informed about an egg donation when the test is requested. If an egg donation is kept secret, the Harmony® Test is not able to deliver a result.
In the case of a vanishing twin, we recommend performing the Harmony® test no earlier than the 15th week of pregnancy, or at least eight weeks after the death of the second fetus9. This is because the placenta of the deceased twin often persists for several weeks, continuing to release cell-free DNA.
The Harmony® Test is not validated for use in pregnancies with maternal aneuploidy, transplant or malignancy.
The Harmony® test can be used for twin pregnancies.
A recent study by Judah et al. (2021) on twin analyses found that the detection and false-positive rates for trisomy 21 are comparable to those of singleton pregnancies9. The publication shows that cfDNA-based screening for the three relevant trisomies in Gemini pregnancies consistently outperforms first trimester screening.
Judah et al.’s meta-analysis was based on the Nicolaides group’s studies with the Harmony® test and publications with MPSS* methods. The detection and false-positive rates for noninvasive prenatal testing (NIPT) in twin pregnancies are9:
| Trisomy | Detection rate | False positive rate | Number of trisomy cases | Number of euploid gemini pregnancies |
| 21 | ||||
| 18 | ||||
| 13 |
Currently, it is not possible to make a statement about sex chromosomal disorders (X/Y analysis), such as Turner or Klinefelter syndrome, for twins.
The table below shows which Harmony® test variant is possible for which pregnancy.
| Singleton pregnancies | Twin pregnancies | More than two fetuses | |
| Harmony® T21/T18/T13 | |||
| Harmony® T21/18/13 + X/Y-Analysis | |||
| sex determination |
Sex determination is possible for both singleton and twin pregnancies. In accordance with the Genetic Diagnostics Act, the fetal sex is communicated from week 14+0 (p.m.) onwards.
In a very small number of cases, the Harmony® Test cannot be performed successfully5 6. This is usually because the proportion of cell-free fetal DNA (cffDNA) in the blood is too low for a result to be obtained. In such a case we recommend blood be taken again at a later point (after 2 weeks) and the Harmony® Test be repeated. In our experience, 50% of the second submissions can then be succesfully evaluated. In case a test result cannot be obtained, a decision has to be made with the patient as to whether further first trimester screening or possibly an invasive procedure should be performed.
Other reasons for test failure include the existence of an egg donation about which was not informed. With the subsequent information of an egg donation, the Harmony® Test can usually obtain a result.
Transplantations or previously unknown (and in general benign) maternal chromosomal disorders can also lead to a failure of the test. For these cases the Harmony® Test is not validated and should not be performed or repeated.
Despite significant improvements over former non-invasive tests, the detection rate for the Harmony® Prenatal Test is not 100%. False-positive results may occure. Therefore the Harmony® Prenatal Test should be regarded as a screening test and not a diagnostic test. A positive (abnormal) result should therefore always be confirmed by a second, diagnostic method (chorionic villus sampling or amniotic puncture) with a subsequent chromosome analysis.
False-negative results are also possible. This is more common for trisomy 13.
The Harmony® Test detects around 30 out of 32 trisomy 13 cases in its current version. A summary of all previous studies on trisomy 13 revealed an average detection rate of 93,8% 7. Trisomy 13 is, however, relatively easy to detect in an ultrasound scan during first trimester screening.
- Ashoor G, Poon L, Syngelaki A, Mosimann B, Nicolaides KH. Fetal Fraction in Maternal Plasma Cell-Free DNA at 11–13 Weeks’ Gestation: Effect of Maternal and Fetal Factors. Fetal Diagn Ther 2012;31:237-243. ↩
- Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013 Jul;33(7):662-6. doi: 10.1002/pd.4119. Epub 2013 May 9. ↩
- Bevilacqua E, Gil MM, Nicolaides KH, Ordoñez E, Cirigliano V, Dierickx H, Willems PJ, Jani JC. Performance of screening for aneuploidies by cell-free DNA analysis of maternal blood in twin pregnancies. Ultrasound Obstet Gynecol. 2015 Jan;45(1):61-6. doi: 10.1002/uog.14690. Epub 2014 Dec 4. ↩
- Gil MM, Quezada MS, Bregant B, Syngelaki A, Nicolaides KH. Cell-free DNA analysis for trisomy risk assessment in first-trimester twin pregnancies. Fetal Diagn Ther. 2014;35(3):204-11. doi: 10.1159/000356495. Epub 2013 Nov 15. ↩
- Lüthgens K, Grati FR, Sinzel M, Häbig K, Kagan KO. Confirmation rate of cell free DNA screening for sex chromosomal abnormalities according to the method of confirmatory testing. Prenat Diagn. 2020 Aug 17. doi: 10.1002/pd.5814. Epub ahead of print. PMID: 32804406. ↩
- Kagan, K. O., Sroka, F., Sonek, J., Abele, H., Lüthgens, K., Schmid, M., Wagner, P., Brucker, S., Wallwiener, D. and Hoopmann, M. (), First trimester screening based on ultrasound and cfDNA vs. first-trimester combined screening – a randomized controlled study. Ultrasound Obstet Gynecol. Accepted Author Manuscript. doi:10.1002/uog.18905 ↩
- Stokowski R, Wang E, White K, Batey A, Jacobsson B, Brar H, Balanarasimha M, Hollemon D, Sparks A, Nicolaides K, Musci TJ.: Clinical performance of non-invasive prenatal testing (NIPT) using targeted cell-free DNA analysis in maternal plasma with microarrays or next generation sequencing (NGS) is consistent across multiple controlled clinical studies. Prenat Diagn. 2015 Sep 1 ↩
- Schmid M et al. Prenatal Screening for 22q11.2 Deletion Using a Targeted Microarray-Based Cell-Free DNA Test.Fetal Diagn Ther. 2017 Nov 8, E-pub ahead of print ↩
- van Eekhout JCA, Bax CJ, Schuurman LVP, Becking EC, van der Ven AJEM, Van Opstal D, Boon EMJ, Macville MVE, Bekker MN, Galjaard RJH; Dutch NIPT Consortium. Performance of non-invasive prenatal testing in vanishing-twin and multiple pregnancies: results of TRIDENT-2 study. Ultrasound Obstet Gynecol. 2025 Sep 6. doi: 10.1002/uog.70015. Epub ahead of print. PMID: 40913805.